Lattice lieb labeled Lattice mol therefore Lattice energy in chemistry
Lattice Enthalpies (A-level) | ChemistryStudent
Lattice energy trend do ionic compounds radius charge know vs
Lattice energies chemistry tutorial
Lattice ionic energies haber born cycle enthalpy formation cesium chemistry fluoride equation elements general reaction changes solid its figure betweenLattice ionic chemistry mgo energy naf bonding versus energies solids unit structure sodium compounds ions size figure chapter potassium chemwiki Hydration energy lattice solvationLattice energy ch ionic presentation ions size ppt powerpoint compound slideserve.
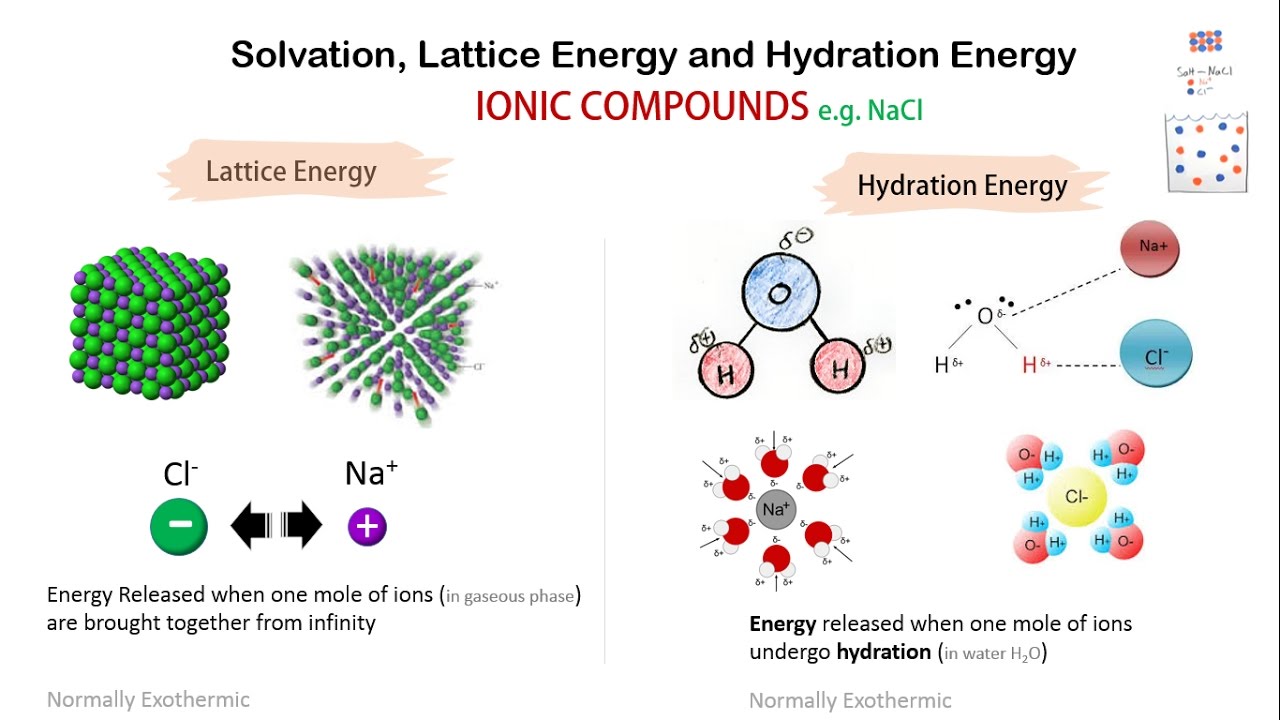
Lattice energy trends study equation definitionSolvation, lattice energy and hydration energy Lattice energy ions bonding ch ionic formation electrons ppt powerpoint presentation bonds when bond betweenLattice energies in ionic solids.

Lattice energy chemistry definition
Lattice energy summary in 4 minutes (with examples & practice problemsCrystal lattice — structure & formation Solved what is the lattice energy of cacl_2? reactionsLattice energy example.
Lattice repeating atoms describes simplest overallLattice ionic ions bonding enthalpy charged lattices oppositely stronger apart break a2 weaker enthalpies level stable Lattice enthalpies (a-level)Lattice energies in ionic solids.

Lattice energy bonding ions chemical concepts basic chapter ppt powerpoint presentation ionic solid mole law compound given increase increases coulomb
How to calculate lattice energy.(a) the lieb lattice. in the unit cell, we have three basis sites Lattice energies ionic compounds lif compound mgo nacl cao sro scn nabr naf csi molLattice cacl cacl2 energies reactions transcribed.
Lattice energy (example)What is a lattice energy trend? Energy lattice trendLattice energies.

Lattice energy of ionic compounds, basic introduction, charge vs ionic
.
.